ELITE CONTROLLER CHRONICLES
Comment on injectable Lenacapavir (SUNLENCA)
From Rebecca Culshaw Smith’s substack
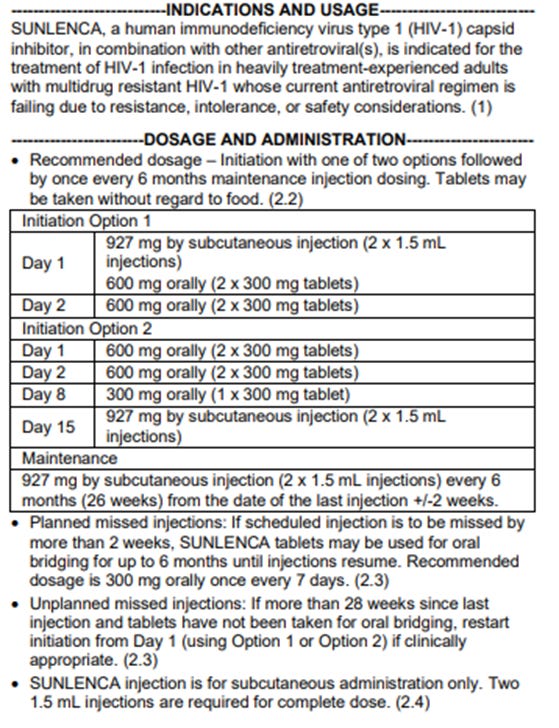
Just a quick heads up, the FDA just approved Lenacapavir for one option for long acting injectable PrEP. Recall that Lenacapavir is a twice yearly injection—how much drug is being administered? Before it was even approved, we saw problems with this drug, including immune reconstruction syndrome (IRIS), and I can’t imagine it will perform particularly well in the future, but time will tell.
Rebecca could simply have looked at prescribing information to answer her question.
My take: the approval of this drug is good news. Not because it works – there is nothing for it to work against – but because the cumulative side effects will be minimal compared to taking daily Truvada or Descovy for PrEP (Pre-Exposure Prophylaxis).
Note that the 927 mg dosage of the twice yearly injection is only 50% more than the daily oral dosage of 600 mg taken for the first week. In other words, a 927 mg injection is supposed to provide protection against HIV for 6 months, but a tablet provides protection for only a day. If you think that makes sense, then you’re probably the type of person who believes the Covid vax has turned you into a spike protein factory. Bless.
The drug is suspended in a PEG solution and injected subcutaneously. Since the rollout of the mRNA vaxes, any mention of PEG raises alarm bells. However, subcutaneous injection, which avoids blood vessels, is much less risky. I did it for 48 weeks, every week, during a period spanning 2009 and 2010. At the time, the therapy to cure “hepatitis C” (a fake viral disease which they diagnosed me with to cover up the fact that my liver was heavily damaged by a dose of the HIV medication Nevirapine) was weekly injections of pegylated interferon alpha. You grab a fold of skin around your waist, insert the syringe into the fold, and inject. It isn’t painful. Most of the other Hep C patients I talked to in a support group described having debilitating severe reactions to the interferon injections, such as terrible nausea, vomiting, fatigue. This was very likely due to reactions to PEG rather than interferon. In any case, I was lucky in being able to tolerate the PEG-interferon injections relatively well. (The other drug I took, Valeant Pharma’s ribavirin, was another story.)
Big Pharma is also trialing for injectable drugs for HIV positive patients. My NHS consultant told me last November that injectable Dolutegravir was in the works. No doubt it will be shown to be effective in drug trials.
In the case of ART, switching from daily pills to injections every six months is in some sense a return to the SMART trial, which I discussed in a previous Elite Controllers Chronicle. But instead of stopping medication for several months until one’s “CD4 count” (another made up number, as you know) falls below 350, they just claim that you remain undetectable for six months. When you go in for your HIV viral load count, they can use the same PCR primers as they would for an HIV negative patient. The reason is that there are no HIV drugs in your bloodstream affecting your ionic concentration.